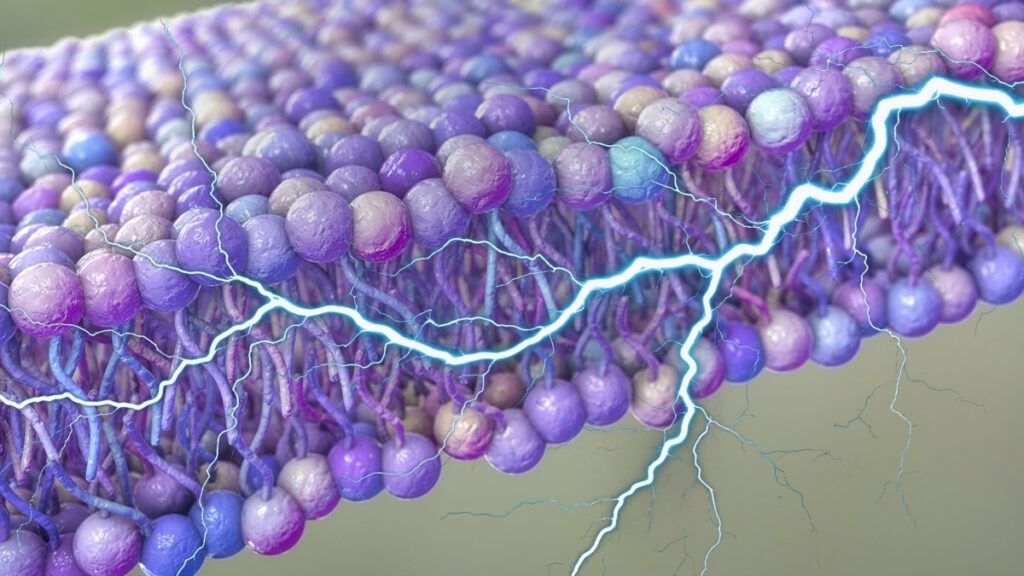
Researchers from the University of Houston and Rutgers University have explored how tiny ripples in the fatty membranes surrounding our cells can generate electrical charges enough to fuel biological processes. These ripples, caused by embedded proteins and ATP breakdown, serve as potential energy sources. The study emphasizes the flexoelectric concept, which relates to generating voltage through strain variations in materials.
The researchers discovered that cellular activity causes membrane fluctuations that can produce transmembrane voltages of up to 90 millivolts—sufficient for initiating neuron firing. This voltage supports ion movement and could impact functions like muscle contraction and sensory signaling, with charges appearing on a millisecond timescale, aligning with nerve cell activity.
The findings suggest that cellular mechanics can enhance energy harvesting and directed ion transport, potentially applicable to larger cell groups. Moreover, the insights could inform designs for artificial intelligence networks and synthesized materials, linking molecular actions to complex information processing and brain function. The study is published in PNAS Nexus.
Source link