Novo Nordisk has filed 14 new lawsuits against organizations involved in illegal practices related to “Semaglutide,” a diabetes treatment. The lawsuits aim to protect patients from unsafe, unapproved drugs that falsely claim to be FDA-approved. These defendants are accused of misleading patients by promoting imitation drugs made with illegal foreign active pharmaceutical ingredients (APIs) and violating state medical laws.
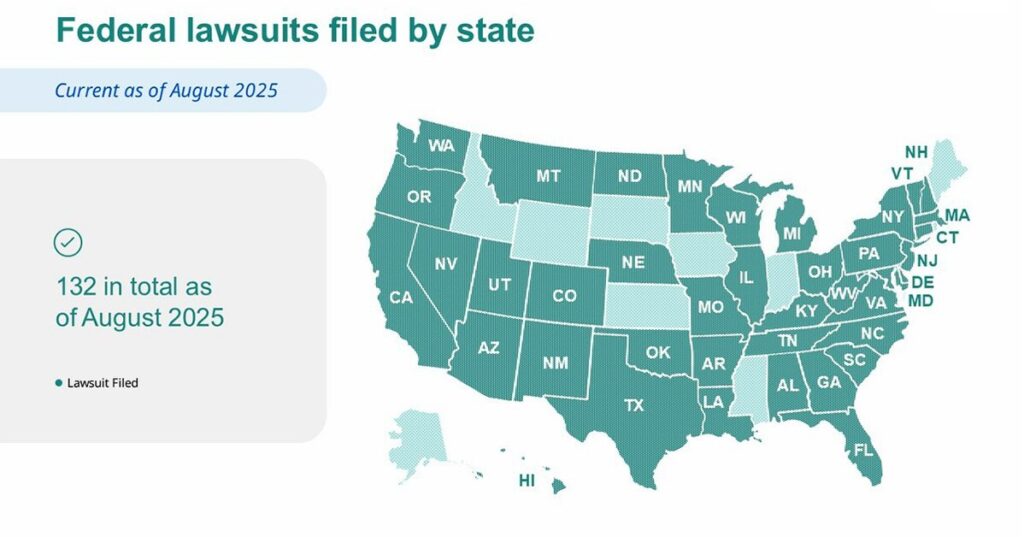
Dave Moore, Vice President of US Business at Novo Nordisk, emphasized the importance of safe treatments and highlighted the risks associated with counterfeit medications. The company has already initiated 132 federal court complaints across 40 states, resulting in 44 permanent injunctions against past defendants. Recent FDA warnings indicate serious health risks from these imitation drugs, which can cause overdoses requiring hospitalization.
A report from the Brookings Institution reveals that many of these products depend on synthetic APIs from unregulated Chinese manufacturers, raising safety concerns. In response, Novo Nordisk has launched awareness campaigns like “Check Before You Inject” to educate patients about the dangers of unapproved drugs and ensure access to authentic, FDA-approved treatments. The company continues to strive for responsible business practices and patient safety in its healthcare solutions.
Source link