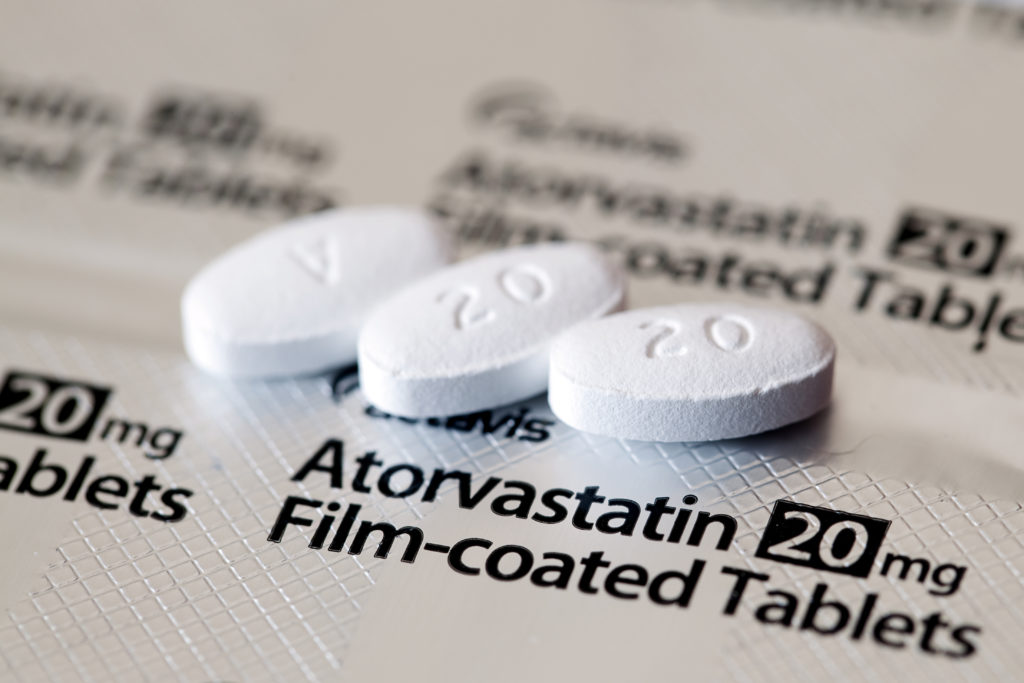
A recent recall of approximately 142,000 bottles of atorvastatin, the generic version of Lipitor, has raised concerns among patients. Atorvastatin, a widely prescribed cholesterol-lowering medication, was found to have quality issues that impacted its dissolution—a crucial factor for drug effectiveness. The recall, issued by Ascend Laboratories, affects medications produced between November 2024 and September 2025 and has been classified by the FDA as Class II, indicating it could cause temporary health effects.
Patients are advised not to discontinue their medication without consulting a pharmacist or healthcare provider, even if their pills are included in the recall. It’s suggested that patients check their prescription labels to identify whether their atorvastatin is from Ascend Laboratories and to consult their pharmacist about alternatives if necessary.
The recall highlights ongoing manufacturing quality issues, particularly with drugs produced overseas. Increasing globalization of pharmaceutical manufacturing, especially in countries like India, poses challenges for FDA oversight. Although measures have been taken to improve inspection protocols, including international collaborations, limitations in testing frequency and resource constraints remain a concern.
Patients are encouraged to pay attention to any changes in drug effectiveness and report these to the FDA, as this could aid in identifying broader manufacturing problems.
Source link